
Leading the World in Production and Supply of Pharmaceutical Raw Materials
In 1980, Nissui successfully developed a high-purity form of EPA, initiating its development as a pharmaceutical raw material. The following year, collaborative research began with a pharmaceutical company, leading to clinical trials. In 1990, the world's first pharmaceutical for the treatment of arteriosclerosis obliterans using high-purity EPA was approved and launched. By 1994, its indications were expanded to include hyperlipidemia, characterized by elevated cholesterol and triglyceride levels in the blood. For over three decades, Nissui has maintained a stable supply of EPA-based pharmaceutical raw materials. Ongoing research continues to highlight EPA's diverse physiological effects, including cardiovascular disease prevention, anti-allergic properties, cancer-suppressing effects, and anti-inflammatory functions.

Figure 1. Fine Chemicals General Factory Kashima No.1 Plant(Fish Oil) and Kashima No.2 Plant (Pharmaceutical) for EPA extraction and advanced purification
Research Approach
Natural fats and oils (neutral lipids from animals and plants) consist of glycerin bonded with various fatty acids (including oleic acid and linoleic acid commonly found in vegetable oils, and EPA and DHA (docosahexaenoic acid) abundant in fish oils). Many types of fatty acids coexist in this form, with sardine oil, the primary raw material, containing approximately 20% EPA. When concentrated, this becomes the health food products commonly available in the market.
| EPA | DHA | Palmitic acid (※3) |
Stearic acid (※4) |
Oleic acid (※5) |
Linoleic acid (※6) |
|
|---|---|---|---|---|---|---|
| Sardine oil as pharmaceutical EPA raw material ※1 | 18.2 | 13.5 | 13.7 | 2.4 | 14.2 | 1.2 |
| Soybean oil ※2 | 0 | 0 | 10.6 | 4.3 | 23.5 | 53.5 |
| Lard ※2 | 0 | 0 | 25.1 | 14.4 | 43.2 | 9.6 |
Figure 2. Comparison of major fatty acid composition (%) in Japanese sardine and edible oils
- ※1Values analyzed by Nissui
- ※2Source: The 5th Revised and Enhanced Standard Tables of Food Composition in Japan, Fatty Acid Composition Tables (2005)
- ※3Palmitic acid
A fatty acid widely distributed in nature; a saturated fatty acid with 16 carbons and no double bonds. - ※4Stearic acid
A saturated fatty acid with 18 carbons, similar to palmitic acid. - ※5Oleic acid
A fatty acid with 18 carbons and one double bond. - ※6Linoleic acid
A fatty acid abundant in plants with 18 carbons and two double bonds. An omega-6 fatty acid with a double bond at the sixth position from the terminal carbon.
To meet pharmaceutical standards, EPA purity must exceed 96.5%. Despite EPA's susceptibility to oxidation making it difficult to handle, Nissui developed proprietary technology combining primary distillation and advanced purification to effectively remove environmental contaminants. This breakthrough enabled the mass production of high-purity EPA exceeding 96.5% purity, which is now widely used as a pharmaceutical raw material.
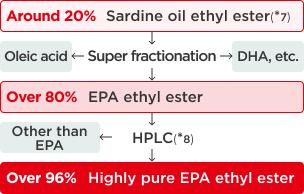
Figure 3. Manufacturing process for high purity EPA
- ※7Ethyl ester
A compound in which ethanol replaces glycerin in the bonding structure (-COO-). This structure remains consistent whether bonded with ethanol or glycerin." - ※8HPLC
High-performance liquid chromatography apparatus that separates compounds using a separation column and pressurized liquid, enabling simultaneous analysis of amino acids.
Research Results
Global demand for EPA continues to grow as research reveals more potential applications. In 2021, Nissui received approval from the U.S. Food and Drug Administration (FDA) and began supplying EPA pharmaceutical raw materials to the United States. We have now established a comprehensive supply system to deliver high purity EPA to pharmaceutical markets throughout Europe, Asia, and beyond.
